Ion or Plasma nitriding is a modern surface heat treatment that sounds very cool and looks even cooler.
But where do the ions come from, and what makes the plasma in ion/plasma nitriding? Let us delve into the mechanics of the process.
Now, let us focus on what exactly happens inside the chamber during the process and delve into the mechanics. To understand it all, we first need to start with the chamber itself.
 |
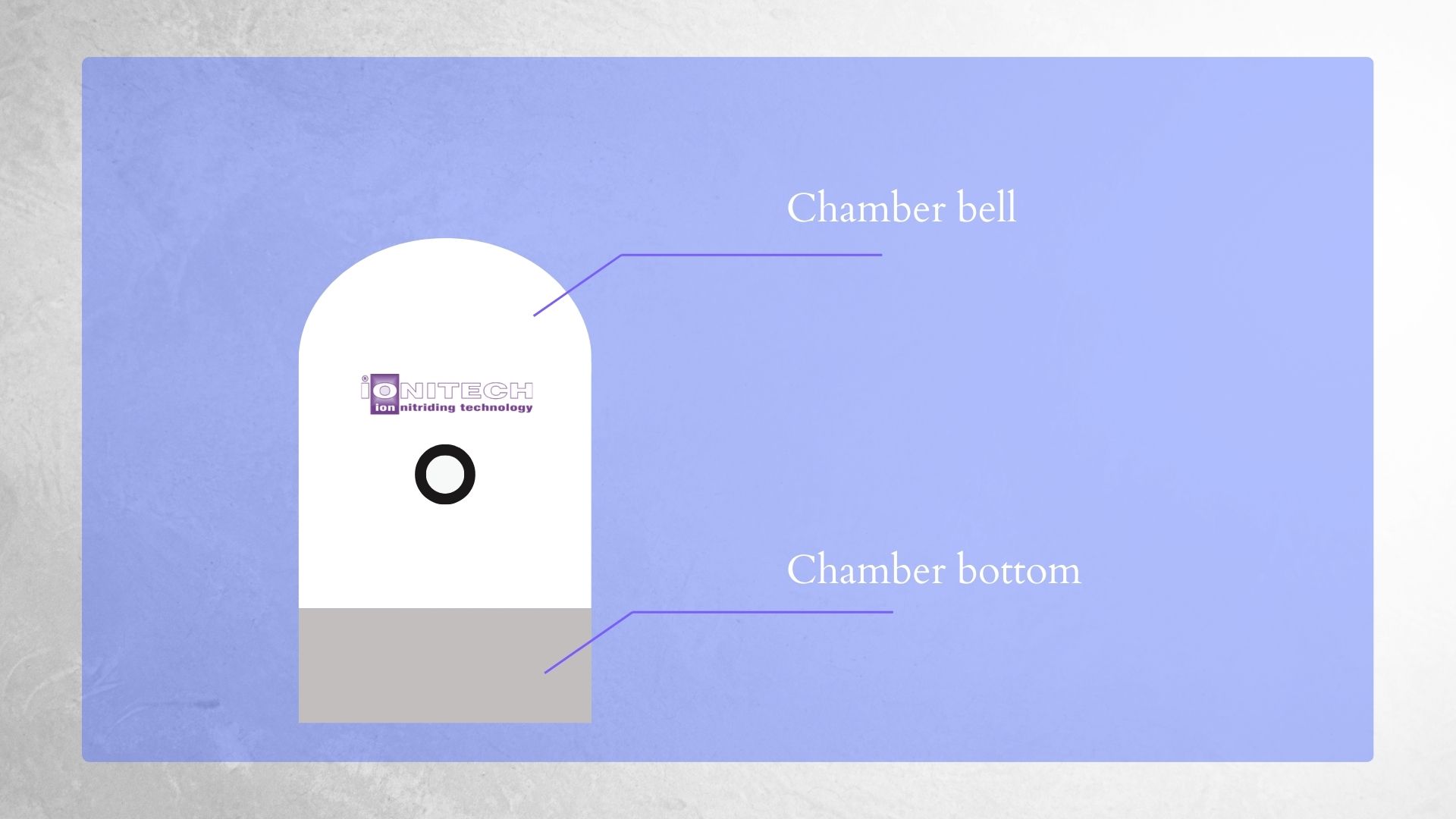 |
| Ion/Plasma Nitriding furnaces by Ionitech. | Schematic of an Ion/Plasma Nitriding furnace |
Inside it, we have a cathode, which is a negatively charged electrode and, in our case, is the main plate. Placing the parts that need to be nitrided on the plate makes these parts negatively charged as well. The walls of the chamber, on the other hand, are an anode—positively charged.
After closing the chamber, a vacuum pump removes the air.
To create the working atmosphere different percentages of hydrogen and nitrogen gases are introduced into the chamber. But how do we get from parts arranged in the chamber to parts in plasma?
Let's start from the beginning.
There are four states of matter—solid, liquid, gas, and plasma. Plasma is the most common state of matter in the universe and it can also be formed on Earth in different ways.
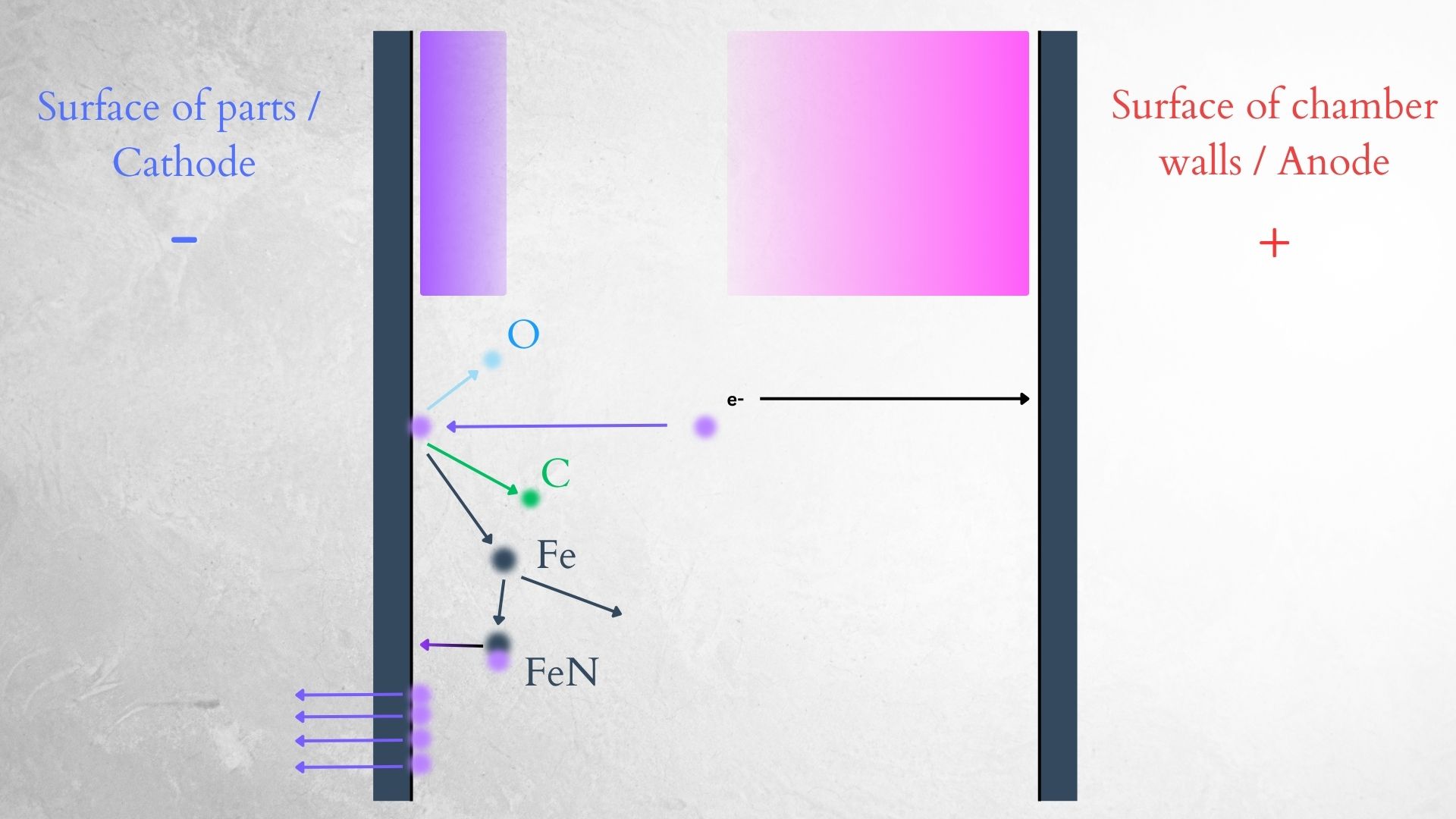
In our case, we are looking at a glow discharge, a type of plasma created by an electrical current that passes through a gas. Voltage is applied between the two electrodes, and some of the gas atoms are ionized by colliding with other atoms. The low pressure in the chamber increases the mean free path, which allows the atoms to gain enough energy from collisions to ionize.

The formed ions and electrons create more collisions, ionizing more atoms and separating more electrons. The electric potential drives the electrons toward the anode, while positive ions are driven toward the cathode—our parts. The collisions of ions with the cathode provide enough energy to release more electrons from the cathode. Once excited, the ions lose their energy fairly quickly. One way they lose energy is by releasing photons, which is the light we see.
Okay, we have created the plasma, but what exactly happens to the parts?
As mentioned earlier, the positive ions move toward the cathode. Ions of nitrogen, hydrogen, and NH radicals bombard the cathode, and two important things happen—sputtering of the surface (removal of surface atoms), and nitrogen diffusion into the material. Nitrogen forms nitrides with iron on the surface of the material, creating the white layer on top, and also diffuses deeper into the steel to form nitrides with iron, chromium, molybdenum, and other alloy elements, creating the nitrogen diffusion layer.
 |
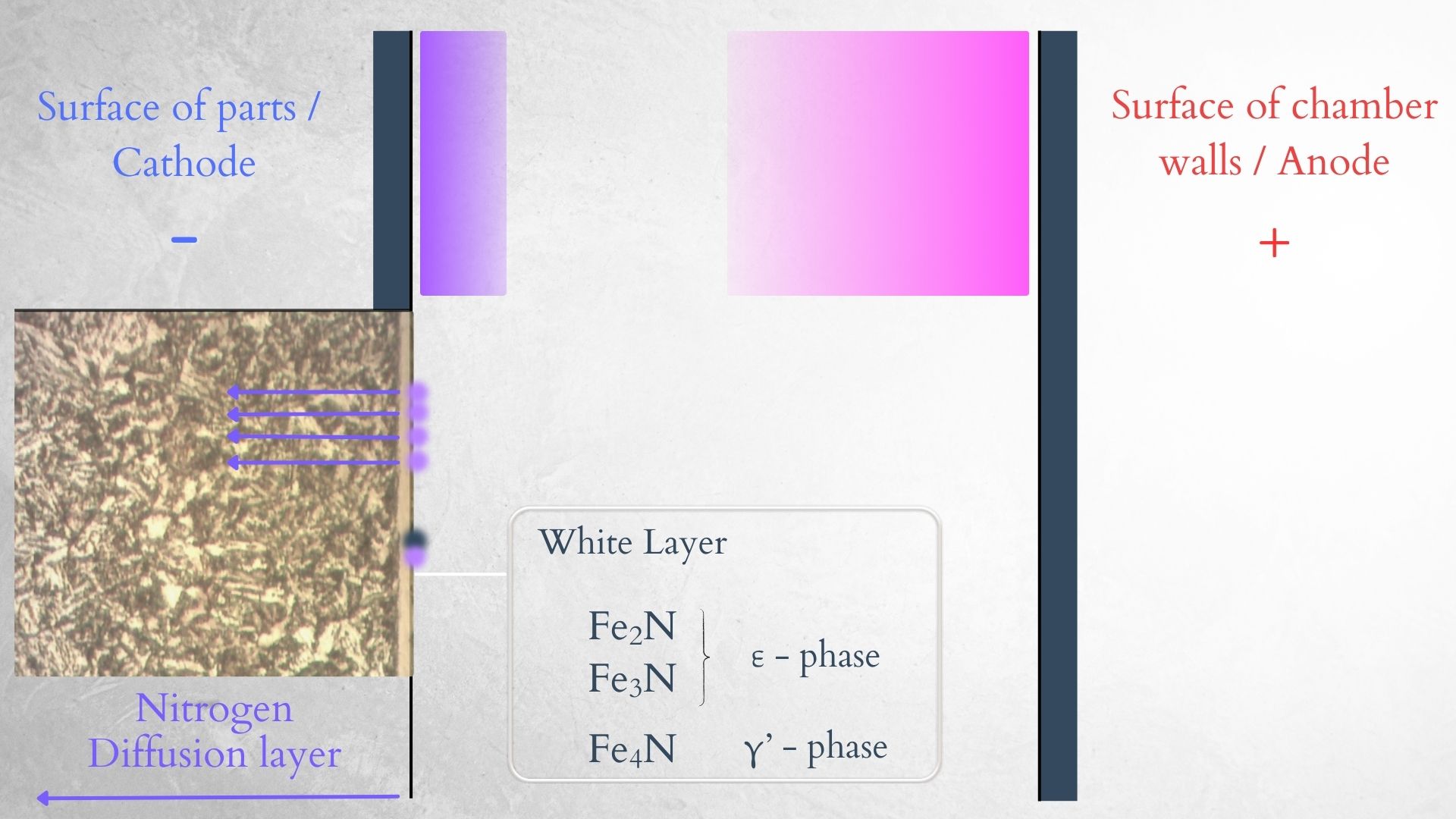 |
| Schematic of ion bombardment - sputtering. | Schematic of nitrogen diffusion and layer formation. |
Ion/Plasma nitriding is a fascinating process. Using the power of plasma to diffuse nitrogen into the metal, it creates a hard, wear-resistant layer that enhances the durability and performance of the parts.
Be sure to check our video for a visual explanation:

 English (United Kingdom)
English (United Kingdom)  Русский (Россия)
Русский (Россия)  Български (България)
Български (България)  Español (España)
Español (España)